This Tiny Spinal Stimulator Could Someday Have a Big Impact on Lower Limb Paralysis
Dinchang Lin’s easily implantable device could make stimulation technology accessible to more patients.
A Johns Hopkins materials scientist and collaborators have developed a tiny device that may hold promise for restoring mobility to those with lower limb paralysis, a condition affecting approximately 1.4 million Americans.
The novel apparatus, a spinal stimulator, can be placed below the injury site through a simple injection, setting it apart from conventional stimulators, which are bulky and must be deployed farther from the nerves that control leg movements.
“The concept behind spinal stimulators is their ability to bypass injured regions, sending essential motor commands from the brain to the spinal region responsible for leg motions. Our innovative approach addresses a key challenge faced by many existing spinal stimulator technologies: achieving precise stimulation and minimal invasiveness,” said member Dinchang Lin, an assistant professor in the Whiting School of Engineering’s Department of Materials Science and Engineering and a core researcher at Johns Hopkins Institute for NanoBioTechnology.
The team’s results appear in Nano Letters: “Injectable Ventral Spinal Stimulator Evokes Programmable and Biomimetic Hindlimb Motion.”
Conventional spinal stimulators are implanted either on the spinal cord’s dorsal surface (facing the person’s back) or directly into the spinal tissue. According to Lin, neither strategy is ideal: The former compromises the implant’s ability to precisely target important nerves, and the latter not only causes damage to the tissue during implantation surgery but also raises biocompatibility issues.
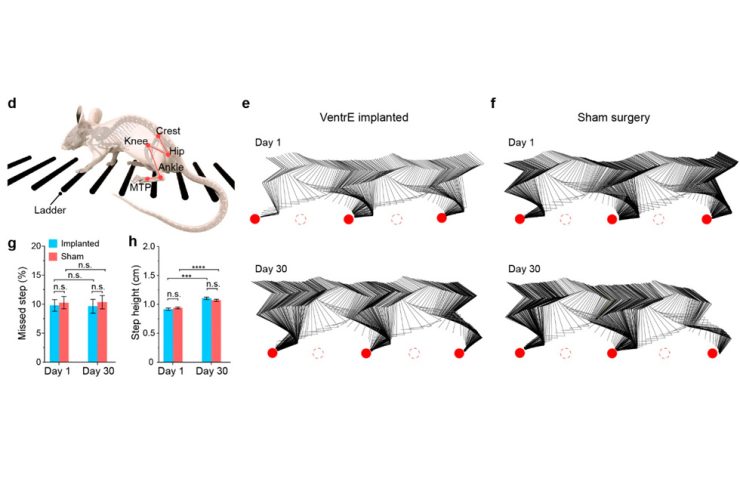
Lin’s team first identified a new site for stimulation, the ventrolateral epidural surface, which is very close to crucial motor neurons in the spinal cord and accessible without surgery. Then they designed a nanoscale, ultra-flexible, and stretchable device that can be inserted via a small injector and a simple syringe pump.
“Applying this new technology in a mouse model, we evoked leg motions using an electric current nearly two orders of magnitude lower than that used in traditional dorsal stimulation. Our stimulator not only enabled a broader range of motions but also allowed us to program the electrode array’s stimulation pattern, which resulted in more intricate and natural leg movements reminiscent of stepping, kicking, and waving,” said Lin, who led the team’s design and selection of the device’s scaffold materials, which was customized to achieve optimal mechanical properties and long-term biocompatibility.
The researchers hope that this technology—if eventually proven safe and effective for use in humans— could someday help restore leg function in people with spinal cord injuries or neuromotor diseases. They also believe that their implantation method with low invasiveness could make it accessible to more people.
“This technology could significantly improve the quality of many patients’ lives, lower the cost of personal care, and help them regain confidence and dignity,” Lin said.
Team members plan to continue work on the device with an eye to eventual human clinical trials.





