Targeting MLL1 Protein Could Slow (or Even Stop) Cancer’s Spread
Cell migration is a critical contributor to the metastasis of cancer cells.
Johns Hopkins researchers have discovered that reducing levels of a key epigenetic protein called MLL1 in tumors can help slow the spread of cancer and potentially inhibit its spread—a finding that suggests that targeting MLL1 could lead to more effective treatments for patients with metastatic cancer.
Their results were published this spring in Science Advances.
“Our results tell us that MLL1 plays a critical role in cancer cell migration. Through targeting this protein, we could develop new approaches to slowing cancer progression and reduce metastasis, improving outcomes for patients with advanced cancers,” said lead author Praful Nair, assistant research scientist at the Whiting School of Engineering’s Institute for NanoBioTechnology, who worked with Denis Wirtz, Theophilus Halley Smoot Professor of Chemical and Biomolecular Engineering and core researcher at the Institute for NanoBioTechnology, on the research.
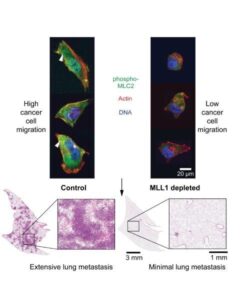
Microscopy image of cancer cell migration in relation to MLL1 depletion
MLL1 (Mixed-Lineage Leukemia 1) is an epigenetic protein that helps control which genes are active in a cell by making changes to different parts of its DNA, which in turn affects how the genes are read and used by the cell.
Nair’s team started by analyzing data from a cancer genome database to identify potential factors that could affect cell migration. They found that the protein MLL1, and its partner Menin, was essential for the migration of cancer cells and that the disruption of this interaction could impair cell migration and metastasis, which causes a majority of cancer deaths.
Cancer cell movement can be triggered by elevated levels of cytokine, which are small proteins that cells use to communicate with each other. While researchers have identified some factors that affect cancer cell migration, they have not yet found any that work by changing gene activity that leads to the production of these cytokines. This is where MLL1 comes in: It is a known epigenetic factor that is strongly correlated with cell migration-related genes.
Using laboratory techniques that mimic the 3D environment of the human body, Nair and Wirtz’s team conducted experiments in which they reduced the activity of MLL1 in cancer cells. They observed that this reduction impaired the cells’ ability to migrate.
“We found that this epigenetic protein controls the production of many of these cytokines which the cells can use to move, sometimes leading them to metastasize and spread to other organs,” Nair said.
In a mouse model of triple negative breast cancer (TNBC), the subtype of breast cancer with the worst prognosis, the depletion of MLL1 from cancer cells reduced the metastatic spread of cancer cells from the tumor and increased their survival. The team also learned that combining an MLL1-Menin inhibitor with other cancer drugs, such as paclitaxel, can both abrogate tumor growth and metastasis, including in cases where cancer has already spread.
“When we administer these drugs to mice that have TNBC tumors, they have been shown to stop the spread of cells cancer cells from the tumor to the lungs,” Nair said.
Latest Posts
-
 Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
-
 Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
-
 Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025


