Researchers examine how dynamic hydrogels affect vascularity in new tissue

Blood vessels are essential to successful tissue and organ function in that they are responsible for delivering oxygen and nutrients throughout the body. It is this same delivery of oxygen, nutrients and blood flow that enables a tumor to grow and spread throughout the body. In a new study published in Cell Stem Cell, researchers at INBT are hoping to discover more about how tissue vasculature can form and adapt in a changing environment. The stiffness of the extracellular matrix (ECM) has long been the topic of regenerative tissue research, especially in the case of cancer cell migration. With this study, Sharon Gerecht’s team has taken the vessel less traveled, by looking instead at the ECM’s responses to “stress relaxation.”
Using a brick wall as a metaphor for tissue formation, endothelial cells, or bricks, send out signaling proteins to interact with the ECM, or mortar, to form the tissue’s structure, or wall. As a result of this signaling, vacuoles, or void spaces, are created between and inside of cells, forming the vessel lumen, or the tubes through which blood flows. The faster blood can flow through newly formed or transplanted tissue, the more successful and stable the tissue will be in its new environment. This is a good thing in the case of transplanted organs or skin grafts but for patients with cancerous tumors, it’s bad news. The nascent, or newly formed, vessels are constantly sprouting and branching through the tissues and the Gerecht Lab’s new research sought to study the effects of the stress or strain of a living, moving environment on the formation of these networks.
While we think of brick walls as being sturdy, immovable structures, Gerecht’s team was interested in how the wall might form in an environment that more closely represents the conditions in a living body. This type of environment is neither rigid nor fluid but viscoelastic – think Jell-O or silly putty. At first, silly putty responds to stress (being squished or pulled apart) by losing firmness and taking a new shape, but after a period of time, the putty relaxes into the new shape and regain firmness.
To develop this viscoelastic ECM, or dynamic hydrogel, the Gerecht Lab tissue engineers developed a new hydrogel scaffold materials platform that mimics aspects of three-dimensional physiological tissue microenvironments (brick walls) and is capable of stress relaxation (like silly putty). Researchers reasoned that the dynamic hydrogel’s ability to respond to the stress of deformation or remodeling can impact how endothelial cells make vasculature.
Co-first author Rahel Schnellmann confirmed that this is exactly what she and her colleagues observed.
“When the dynamic hydrogel is deformed, the cells within are rearranged and can create new signaling pathways with other cells and form new vascular networks,” said Schnellmann.
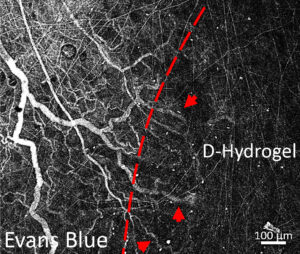
Blood vessels growing into the viscoelastic dynamic hydrogel.
This rearrangement of the cells within the dynamic hydrogel is remarkable because a stiffer, more static hydrogel, commonly used in studies involving tissue regeneration, does not yield the same downstream signaling between cells and speedy vasculature formation.
The research also revealed the new vasculature form quicker within a dynamic or stress relaxing hydrogel and can pass fluid through and grow larger than in non-dynamic hydrogels. Endothelial cells can then communicate more efficiently with cells in new tissue growth or an engineered tissue graft placed in the existing environment. The faster formation of a network in the ECM can occur, the more efficient the integration process for the new tissue into the new environment. This characteristic could also prove to be essential to the development of treatments for slowing down vascular growth in tumors and slowing the spread of cancer through the body.
“If you want effective tissue remodeling and vascularization, the tissue has to have a high stress relaxation property, it has to be dynamic,” explained Schnellmann, “otherwise, all of your processes are extremely slowed down.”
Faster cell signaling and vascularity formation would benefit research on a variety of topics from wound healing, faster integration of transplanted organs, how cancer spreads throughout the body and more.
The paper, “Hydrogel network dynamics regulate vascular morphogenesis”, will appear in the November issue of Cell Stem Cell and is online this week.
Read the study on Cell Stem Cell and visit Gerecht Lab’s website for more information on their ongoing research at INBT.
Story by Amy Weldon