New Screening Platform for Uncovering Potent Lipid Nanoparticles to Deliver Gene Medicine
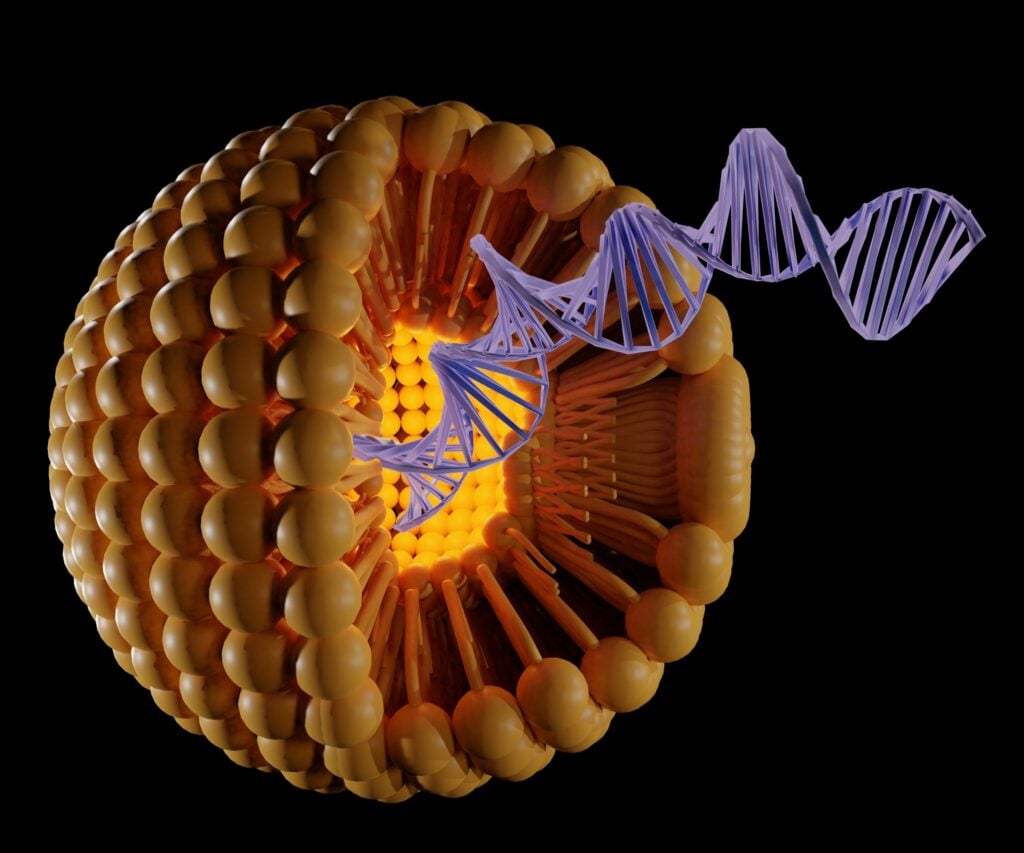
The success of COVID-19 vaccines has shown tremendous potential for using gene medicine to prevent viral infections. One reason for the vaccines’ success is attributed to the use of lipid nanoparticles (LNP) as a vehicle to deliver messenger RNA (mRNA) to cells to generate and boost immunity. LNPs have become increasing popular for delivery of other gene-based medicines such as small interfering RNA, microRNA, or DNA; but each gene medicine requires a tailored LNP design. To engineer the best LNP design for different therapeutic genes, Johns Hopkins biomaterials engineer Hai-Quan Mao and his collaborators created a multi-step platform screening platform that enables high-throughput selection of the optimal LNPs.
A crucial feature to effective treatments is how long a gene medicine lasts once it is expressed by the cell. In COVID-19 vaccines, the mRNA are instructions to make a harmless virus-specific protein, which helps the body recognize the virus and generate an immune response upon viral exposure. Such process of converting the instructions encoded by the gene into a functional product is called expression. When LNPs deliver mRNA to cells, its expression starts declining rapidly within the first 24 hours. A promising alternative is plasmid DNA (pDNA). This double-stranded circular DNA is more stable and can last for seven days, which can substantially extend its therapeutic effect. However, effective methods for screening multiple lipid components for the best pDNA packaging and delivering design are lacking.
“The optimal LNP design for a particular gene medicine is highly dependent on the therapeutic payload it carries and the cell type one wants to target. This makes the screening process extremely challenging,” said Hai-Quan Mao, director of the Institute for NanoBioTechnology (INBT) and professor in the Departments of Materials Sciences and Engineering and Biomedical Engineering.
Yining Zhu, an INBT researcher and biomedical engineering PhD student mentored by Mao and Sashank Reddy, INBT associate director and assistant professor in Departments of Plastic and Reconstructive Surgery and Biomedical Engineering, led a team to identify the best LNP design for pDNA delivery to liver cells. Their platform screens LNPs in a stepwise manner using strategies that address the physiological barriers an LNP encounters as it navigates through the body to reach the target cells, where it must then enter and release its therapeutic payload. The platform helped the team identify the most effective LNPs from a library of over 1,000 combinations.
The team published their findings in Nature Communications. Their study is the first to develop a streamlined process to systematically investigate LNP compositions for pDNA delivery in
cultured cells and mouse models. Not only that, the platform helps reduces the time and cost it takes to develop LNPs, and they doubled the duration the genetic medicine is expressed. This approach can be easily adapted for other gene therapies.
“This platform is versatile in that it is not merely limited to pDNA delivery but can be easily extended to the development of LNPs for a wide range of therapeutic gene payloads such as DNA, mRNA, guide RNA, and microRNA, as well as alternative delivery routes such as oral, intramuscular injection, or inhalation method,” said Zhu.
In collaboration with Sean Murphy, associate professor at the University of Washington, and his group, the joint team is now leveraging LNPs identified with the platform to develop a malaria vaccine that targets the disease-causing parasite during its lifecycle in the liver. The screening platform can help accelerate other LNP product innovation to push the bounds of gene medicine, vaccine development, and other novel therapeutics.
This research was funded the National Institutes of Allergy and Infectious Diseases at the National Institutes of Health.
Story by Zhenhui (Christine) Wei and Gina Wadas. Read more about this research on the Hub.





