New “Carrier Cocktail” Packs Powerful One-Two Punch Against Solid Tumors
Johns Hopkins team combines radionuclide-carrying antibodies and nanoparticles to enhance radiation delivery.
Researchers from the Whiting School of Engineering’s Department of Chemical and Biomolecular Engineering and Institute for NanoBioTechnology have found that combining two alpha-particle-carrying cancer treatments significantly enhanced their effectiveness in battling solid tumors compared to each therapy individually. Their results appear in the European Journal of Nuclear Medicine and Molecular Imaging.
Alpha-particle-carrying cancer treatments are a form of therapy that uses radioactive isotopes that emit alpha–particles to selectively kill cancer cells while preserving surrounding healthy tissue. The particles damage the cancer cells’ DNA, crippling their ability to replicate and spread.
“Alpha-article treatment has been most effective on tumors with many markers on the surface of their cancer cells, which act as targets for the short-range alpha-particles to hit. However, the treatment has been less useful on tumors with minimal cell markers, and that is the problem that we set out to solve,” said study co-author Rajiv Ranjit Nair, a doctoral student in the Whiting School of Engineering’s Department of Chemical and Biomolecular Engineering.
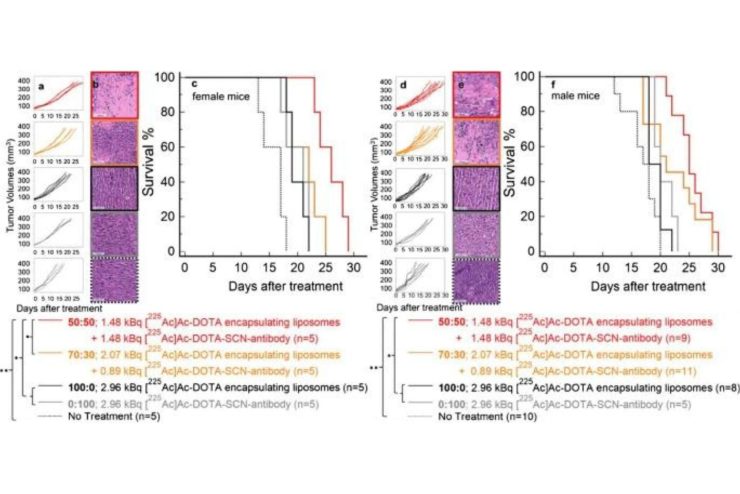
To address this issue, the team combined two types of treatment delivery systems: antibody-radioconjugates—antibodies attached to radioactive particles that bind to cancer cells near blood vessels—and liposome nanocarriers—minuscule particles loaded with radioactive emitters that can penetrate deeper into tumor areas farther from blood vessels. Their goal was to evenly distribute the alpha–particles within tumors with fewer cell markers.
“Combing both carriers allows for more area of the tumor to be covered, increasing the likelihood of hitting and killing more cancer cells,” said team leader Stavroula Sofou, associate researcher for the Institute for NanoBioTechnology, professor of chemical and biomolecular engineering and member of SKCC’s Cancer Invasion & Metastasis Program.
The researchers tested their combined approach on mice with breast and pancreatic cancer and found the new carrier cocktail significantly improved the treatment.
“In the breast cancer models, the combined carriers reduced the tumor by 73% compared to the traditional method. In pancreatic cancer models, the survival time of mice increased significantly,” said Nair. “The combined treatment also prevented the formation of spontaneous mestastases: secondary tumors that often develop when the cancer spreads to other parts of the body.”
Additionally, the researchers found that administering a low dose of the chemotherapy drug gemcitabine before using the dual-treatment further enhanced its efficacy, both stopping tumor growth and prolonging survival.
The team believes that its findings may open new treatment possibilities for patients with tumors that previously would not have responded to alpha-particle therapy. They say that their approach’s ability to improve the distribution of alpha-particles throughout tumors could also make existing treatments more effective, reducing the need to develop new targeting drugs for different types of cancer.
“With the improved distribution of particles, this treatment’s effectiveness can be extended to various types of solid tumors,” said Sofou. “This strategy allows for a more uniform delivery than we previously relied on and has the potential to lead to better treatment responses.”
Study co-authors include Aprameya Prasad, Omar Bhatavdekar, and Aira Sarkar—all of the Department of Chemical and Biomolecular Engineering and the Institute for NanoBioTechnology—and Kathleen L. Gabrielson, of the Johns Hopkins School of Medicine’s Department of Molecular and Comparative Pathobiology.
Story by Emily Flinchum
Latest Posts
-
 Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
-
 Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
-
 Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025


