Measure for Measure: Steps Toward Standardization in the Field of Cell Mechanics
A cell’s properties—the way it moves, its shape, its texture, and its stiffness—have an enormous impact on human development, the immune response, and the progression of cancer.
But researchers studying those cell mechanics often wind up with different results from their colleagues’, leading to confusion and delaying potential breakthroughs in cancer treatment and immunotherapy.
Denis Wirtz, Theophilus Halley Smoot Professor in the Department of Chemical and Biomolecular Engineering, and colleagues recently found that the discrepancies were due to different measurement techniques. In a paper published in Nature Methods in July, they analyze measurements obtained by some of the most common methods and discuss the importance of selecting an appropriate technique for the biological issue being investigated.
“We wanted the paper to be a practical guide for the non-expert to decide which technique to use for which purpose, and to make cell mechanics a little more prominent,” says Wirtz, who is also vice provost for research for Johns Hopkins, director of the Johns Hopkins Physical Sciences-Oncology Center, and co-director of the Cancer Nanotechnology Training Center. He is a co-founder and former associate director of the Johns Hopkins Institute for NanoBioTechnology.
Cell mechanics, a field that has grown rapidly in the last decade or two, is crucial in cancer research; there is a strong correlation between the stiffness of tumor cells and how readily they metastasize. More generally, it offers insight into how cells are actually behaving, rather than what they are capable of doing. As the field has grown, a wide range of approaches to measurement has developed, so Wirtz says it’s time to “push pause” and standardize the most appropriate techniques. Unifying the maturing field around a common set of approaches will make cell mechanics more valuable to the larger cancer research community, he says.
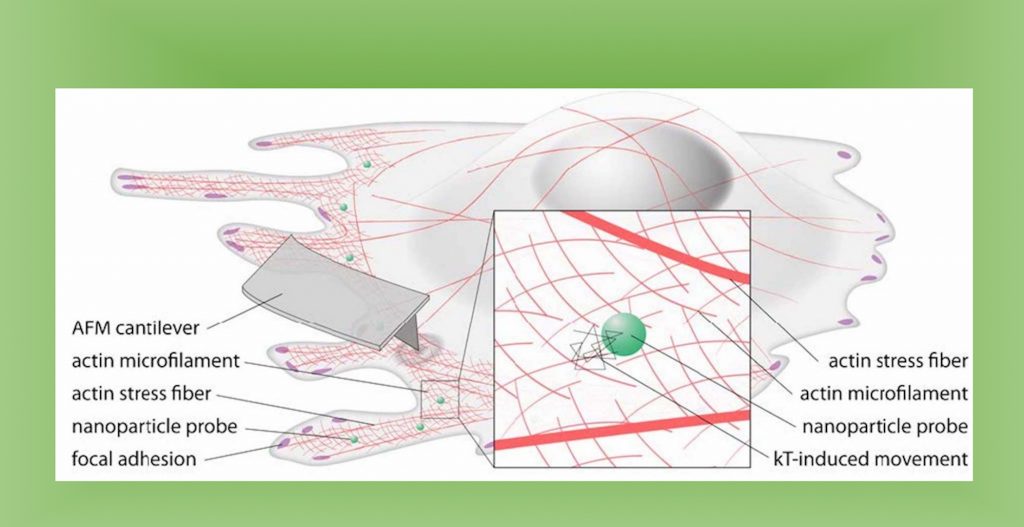
The researchers compared measurement methods by probing the same type of cell using multiple techniques, which required minimizing biological variation. With co-authors across the U.S., France, and Germany, this was not an easy feat, but all researchers used the same line from the same batch of cells. Measurement methods included atomic force, microscopy, magnetic twisting cytometry, particle-tracking microrheology, parallel-plate rheometry, cell monolayer rheology, and optical stretching. In at least one example with breast cancer cells, they found measurement variations up to 1,000-fold. Sources of variation included the level of applied mechanical stress, the rate of deformation, the geometry of the probe, the location probed in the cell, and the extracellular microenvironment.
The result of the study is a catalog of measurement variations among common techniques, paving the way for standardized protocols that yield consistent results across researchers and a clear way forward for the field. “Having co-authors in different countries was a signal that this is not a single institution’s version, but that it’s inclusive from the beginning,” Wirtz says.
After laying the groundwork with cultured cells, Wirtz says the next step is to do similar research in vivo, in acknowledgement that the macro environment has its own impact on the physical properties of cells. In the very long term, Wirtz envisions cell mechanics assessments—with simplified measurements—as one of many tests offered to patients in clinical settings.
This article was originally posted by the Department for Chemical and Bimolecular Engineering.