Engineers ‘hijack’ natural cell process to deliver large drug molecules
Discovery could boost effectiveness of cancer and other medications
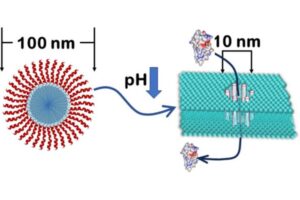
Image depicting the modified peptides forming nanopores to allow larger molecules through a cell’s membrane.
Scientists at Johns Hopkins and Tulane University have discovered a way to transport large molecules into cells by opening tiny compartments, potentially enabling the direct delivery of antibodies and cancer-fighting medications. Their results appear in ACS Nano.
“Usually, macromolecules like certain medications for cancer cannot go directly into the cell because of the barrier imposed by the membrane, the wall that surrounds the cell,” says Kalina Hristova, core researcher at the Institute for NanoBioTechnology and professor of materials science and engineering at Johns Hopkins Whiting School of Engineering. “We were able to ‘hijack’ a process called endocytosis, which is how cells naturally let molecules in, by discovering peptides that make the pores that allow macromolecules inside a cell.”
Supported by a $1.6 million grant from the National Institutes of Health, the researchers examined these peptides, which work by forming holes called nanopores in membranes during endocytosis. They found that a specific peptide, called pHD 108, can be chemically altered to become more effective in forming the nanopores. After adding fatty acid chains to either side of the peptide, they found that the nanopore-forming activity increased.
“These peptides activate when the pH level, which measures acidity, dips inside of compartments called endosomes in cells, causing them to assemble and form a nanopore that allows macromolecule drugs out of the endosome and into the cell,” says Hristova.
After the researchers modified the peptide, their experiments showed that specific large molecules could reach the cell’s interior after the endocytosis process – including cytotoxic enzymes for cell death and fluorescent proteins for imaging, which they successfully delivered into the cell.
“Normally, such molecules are broken into smaller pieces because of the destructive enzymes inside the endosomes. Without the modified peptides that we discovered, these molecules fail to reach their intended targets, making them ineffective.” says Hristova
Next, the researchers want to try this with different macromolecules, like cancer medications or antibodies.
“Antibodies are prevented from entering the cell because of its outer membrane. Now we want to use this method to see if they can go into cells and possibly treat cancer within them,” says Hristova.
Hristova and Hopkins student Kenyon Bell were joined by Professor William Wimley of the Department of Biochemistry and Molecular Biology at Tulane University and his students Eric Wu, Ains Ellis, and collaborators Daniel L. Moss, and Samuel J. Landry.
Story by Conner Allen
Latest Posts
-
 Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
-
 Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
-
 Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025


