A Tube with a View: Engineers Build a Precision Model of a Human Fallopian Tube
New tool could advance research on fertility, cancer, and more.
Johns Hopkins engineers have created an innovative and highly accurate lab-grown model of the human fallopian tube— an innovation that promises to advance research on ovarian cancer, gynecological diseases, and fertility. By closely replicating the structure and function of the natural organ, the model opens new avenues for studying a wide range of biological processes and diseases.
“Combined assembloid modeling and 3D whole-organ mapping captures the microanatomy and function of the human fallopian tube,” appears in Science Advances.
“Our model provides a powerful and accurate new tool to study both healthy and diseased fallopian tubes in the lab,” said team member Denis Wirtz, professor of chemical and biomolecular engineering at the Whiting School of Engineering and core researcher at its Institute for NanoBioTechnology. “The idea is to use it to understand infertility and cancer development better, test new treatments, and potentially apply our validation methods to create models of other organs.”
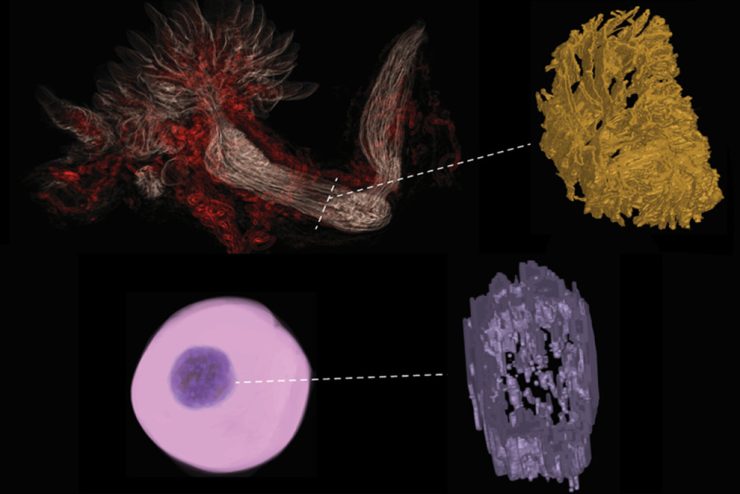
The team set out to create an optimized platform for studying fallopian tube health while also providing a framework for generating accurate in vitro models of other organs. They began by arranging different types of cells into a 3D shape modeled on that of a living fallopian tube, with its distinct layers of epithelial cells—which line the inside of the tube to move eggs along—and stromal cells—which support the tube’s structure.
Using an analytical technique called mass spectroscopy, the team compared their model’s protein composition and architecture with that of a living fallopian tube, identifying and quantifying individual protein molecules by their charge and mass. They then used CODA imaging—a technique developed by Wirtz’s lab—to analyze their lab-grown model alongside real human fallopian tubes. CODA scans samples at high magnification, creating highly detailed tissue maps, which are then analyzed using advanced computational methods to assess their similarities and differences.
Insights gained through CODA allowed the team to fine-tune their model, repeatedly testing and adjusting the model’s combination of cell types, the structure of the scaffold between the cells, and the physical structure of the tissue, until it closely matched those of real fallopian tubes.
The team said the resulting model could be used for a variety of research, from modeling ovarian cancer and ectopic pregnancy to studying how hormones influence fallopian tube function and embryo implantation.
“This model opens new avenues for understanding fallopian tube health and developing targeted treatments,” Wirtz said.
This research was supported by the National Cancer Institute, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Cancer Institute Ovarian Cancer Specialized Program of Research Excellence (SPORE) at Johns Hopkins University, National Cancer Institute Cervical Cancer Career Development SPORE at Johns Hopkins University, and National Institutes of Health U01.