Self-Assembling Hydrogel Awakens Immune System for Better Cancer Immunotherapy
The immune system has various means to detect infectious invaders, like bacteria and viruses. and non-infectious ones, like cancer. Some cancers, however, can evade immune surveillance and even switch off our immune system. To assist the immune system, several cancer immunotherapies have been developed and have shown beneficial for many cancer patients, including those in advanced stages and with metastatic cancers. These therapies work by either stimulating the immune system’s normal defenses or using lab-made materials that mimic immune system functions.
For patients with tumors that lack immune cells and other cancer fighting molecules in and around the tumor, also known as cold tumors, the effectiveness of cancer immunotherapies can be low. To help these patients, Honggang Cui, Feihu Wang, Hao Su, and other colleagues, investigated a possible combination chemo-immunotherapy approach using a known chemotherapy drug called camptothecin (CPT) and activating a cell signaling pathway called stimulator of interferon genes (STING), which stimulates the immune system.
Cui is a core faculty member at the Institute for NanoBioTechnology (INBT) and associate professor in the Department of Chemical and Biomolecular Engineering at the Whiting School of Engineering. Wang is a postdoctoral fellow in Cui’s lab and Su is a former PhD student in the Cui lab and is currently a postdoctoral fellow at Eindhoven University of Technology.
CPT is often administered intravenously. However, this drug delivery method often has off-target effects, meaning that the drug can interact with anything it encounters in body besides the tumor, which creates side effects. It also does not allow for long-term release of the drug and the contents lose their potency as it circulates the body before it reaches the tumor. To address this, the team converted CPT into a self-assembling hydrogel, a gelatin-like substance. As a hydrogel, CPT could be administered directly into the tumor and would provide long-term drug release by degrading over a two-month period.
The hydrogel form also allows the team to incorporate cyclic dinucleotide c-di-AMP (CDA), a molecule that activates the STING pathway. When CPT, the chemotherapeutic drug, kills the cancer cells, it inflames the tumor microenvironment and attracts immune cells to the tumor sites. This process works synergistically with the STING pathway activation, provoking a stronger immune response and helping the immune cells penetrate the tumor to destroy it. Now that the tumor has the assistance of the immune system, it is referred to as an immune-stimulating tumor, and immune-stimulating tumors allow for immunotherapies to be more effective.
“The hydrogel we designed has a twofold role. First, it is a drug that helps create an immune responsive environment, and secondly, it a delivery medium that allows for the sustained release, over a two-month period, of the drug,” said Cui.
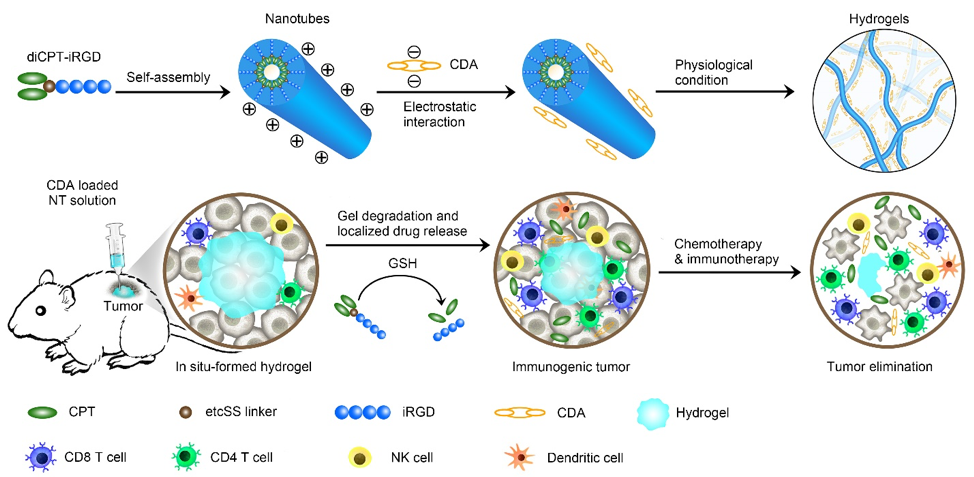
The illustration depicts the hydrogel’s design, the delivery method, and immune response. Image credit: Feihu Wang and Zongyuan Wang, Johns Hopkins University
The team tested their therapy on mouse models with subcutaneous brain, breast, and colorectal tumors. Their results showed near tumor regression in most of their models with no obvious side effects. Not only that, when the tumors were destroyed, their contents leaked into the surrounding environment. This caused inflammation, which further stimulated an immune response to the area by activating other immune cells such as natural killer cells, T cells, and dendritic cells.
After the primary tumors were treated, the researchers reintroduce the same cancer back into the mice, a process known as rechallenging the tumor. Remarkably, the tumors did not regrow. By delivering CPT and CDA via the hydrogel form directly into the primary tumor, the immune system produced memory T cells, which provides the immune system with long-term memory and surveillance for the same tumor in the future.
The team published their finding in a recent journal article in Nature Biomedical Engineering and it offers promising results for patients with cold tumors, but it can also help with tumor recurrence and metastasis.
Story by Gina Wadas
More Information
More recently, Cui, Wang, and coauthors reported on the use of a drug-based supramolecular hydrogelator for local delivery of immune checkpoint inhibitors to boost the host’s immune responses against tumor. They found that supramolecular hydrogels could serve as a reservoir for sustained co-release of CPT and anti-PD-1 antibody, resulting in an immune-stimulating tumor microenvironment for boosted PD-1 blockade immune response, which elicited robust and durable systemic anti-cancer immunity, inducing tumor regression and inhibiting tumor recurrence and metastasis.
Latest Posts
-
 Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
Cellular building blocks may enable new understanding of the body’s “machinery”
December 19, 2025
-
 Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
Biomedical Engineer Jamie Spangler Receives President’s Frontier Award
December 15, 2025
-
 Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025


