Thickness of Cell Culture Media Matters to Improve Costly, Important Gene Therapy Process
A team of Johns Hopkins researchers discovered that the thickness of fluid in cell culture media, the engineered environment for growing and researching cells in a laboratory, plays a critical role in an important and costly process in gene therapy development.
Gene therapies are promising treatments for many acquired and congenital diseases. However, part of what makes developing these therapies so costly to manufacturers and patients is the transfection process. It involves inserting nucleic acids, such and DNA and RNA, into cells to alter gene expression or produce specific proteins. To achieve this, the nucleic acids are loaded into carriers, like nanoparticles or synthetic virus, and delivered to the body where they induce biological responses in specified cells.
While there have been advancements to the transfection process, it still has challenges that make it less efficient, and expensive. This leaves much room for scientists to improve the process to make gene therapies accessible to more patients. Hai-Quan Mao, director of the Institute for NanoBioTechnology and professor of materials science and engineering, and his team studied an overlooked biophysical property that offers promising results: viscosity. They discovered that when the viscosity closely resembled the fluid thickness in the human body, transfection improved.
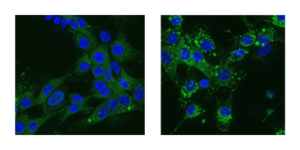
Cells (blue with faint green contour) transfect more lipid nanoparticles (bright green spots) at a higher efficiency in culture media with a viscosity similar to those found in the human body (right) compared with standard culture medium (left).
“Most cell culture media used in transfection are about the consistency of water. By adjusting the viscosity to resemble that of blood and interstitial fluid, we substantially improved transfection efficiency,” said Mao.
Lead by materials science and engineering PhD student, Jingyao Ma, and biomedical engineering PhD student, Yining Zhu, the team systematically adjusted cell culture media viscosity and tested its effect on transfection of various cell types and carriers, including lipid nanoparticles, polyplexes, adeno-associated vectors, and lentiviral vectors. Each carrier showed a ‘goldilocks zone’ where maximum transfection occurred depending on the media viscosity. Overall, transfection improved two- to sixty-fold across all the carriers tested when the media resembled the viscosity of bodily fluids.
Their research is published in Nature Chemical Engineering.
The team’s results have broad applications. For instance, it could be integrated into existing gene and cell therapy manufacturing to make more products using the same amount of resources. Reducing the manufacturer’s production costs while increasing their yield can make more treatments available at a lower cost to patients. Additionally, the research could improve the predictive power of preclinical studies, helping to accelerate the development and approval of new gene therapies.
“This research has the potential to transform the landscape of gene therapy by making it more efficient, scalable, and accessible to a broader population,” said Ma and Zhu.
Latest Posts
-
 Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
-
 Micro Grippers: David Gracias Builds Micromachines That Fold, Stick, Swim, and Sense—All Inside the Human Body.
November 20, 2025
Micro Grippers: David Gracias Builds Micromachines That Fold, Stick, Swim, and Sense—All Inside the Human Body.
November 20, 2025
-
 A bold new approach to autoimmune diseases
November 19, 2025
A bold new approach to autoimmune diseases
November 19, 2025


