New gene editing treatment being developed by researchers
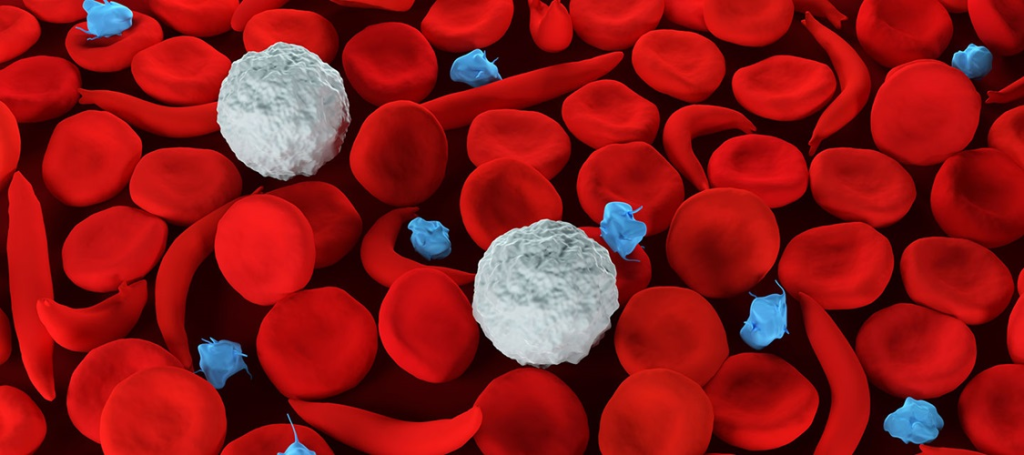
Photo courtesy of Johns Hopkins Medicine
Sickle cell disease is caused by an inherited genetic mutation. Current gene therapies are complex, time-consuming, and risky, with some clinical trials revealing serious side effects such as infertility or blood cancer.
To address these challenges, Johns Hopkins researchers have developed special nanoparticles that can send gene treatment directly to various types of cells in bone marrow to correct the disease-causing mutations. Their results appear in Nature Nanotechnology.
“This gene editing approach would allow patients to receive the medicine through a transfusion This avoids the lengthy, difficult process of many current gene therapies, decreasing the burden on patients and the health care system while minimizing treatment side effects,” said study lead author Xizhen Lian, an assistant research scientist affiliated with the Johns Hopkins Whiting School of Engineering’s Institute for NanoBioTechnology and the Johns Hopkins School of Medicine.
The research team, which included scientists at the University of Texas Southwestern Medical Center, St. Jude Children’s Research Hospital, Harvard University, and Johns Hopkins School of Medicine, used CRISPR/Cas and base editing gene-editing techniques in a mouse model of sickle cell disease to activate a form of hemoglobin and correct the sickle cell mutation. The team also found the approach effective in targeting leukemia cells.
“One challenge we encountered is that the stem cell population is very small; only 0.1 percent of cells in bone marrow are stem cells. They are also protected in a micro-environment that can prevent the delivery of drugs from circulation,” Lian said.
Lian and the team solved this problem by adding a special fat molecule into their tiny delivery particles. This new molecule helped the delivery particles find and strongly attach to the stem cells, delivering important gene therapy.
The team’s next step is to optimize this technology on a humanized animal model that can better mimic clinical scenarios, as they are currently working solely with rodent blood cells and components. Humanized animal models have been genetically modified to express human genes, cells, and proteins, allowing researchers to study human diseases in a living system that closely resembles that of humans.
“Our approach promises to help patients avoid invasive treatment procedures, which will significantly reduce the side effects of blood cancer because there is no random gene insertion into the patient’s genes. We are targeting a specific gene that causes the disease and that’s it,” Lian said. “The only way to cure such genetic diseases is to correct the genetic mutation in the stem cell populations.”
Latest Posts
-
 Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
Johns Hopkins Postdoc Named in Forbes `30 Under 30′ List
December 8, 2025
-
 Micro Grippers: David Gracias Builds Micromachines That Fold, Stick, Swim, and Sense—All Inside the Human Body.
November 20, 2025
Micro Grippers: David Gracias Builds Micromachines That Fold, Stick, Swim, and Sense—All Inside the Human Body.
November 20, 2025
-
 A bold new approach to autoimmune diseases
November 19, 2025
A bold new approach to autoimmune diseases
November 19, 2025


